They are developing new treatments to mitigate cardiovascular pathologies induced by obesity and diabetes and unique trans-tissue drug delivery systems for localized treatments to prevent diabetic foot ulcers. Their efforts are also directed at developing a lung treatment to mitigate chronic infection and inflammation in the lower airways of the lungs via localized deep-tissue drug delivery. Past research from the Pulakat Lab identified cardiac-specific cytokine and microRNA markers associated with the progression of diabetes and in response to treatment with Rapamycin, an anti-aging drug. They also elucidated the structure-function relationship of cardiovascular reparative Angiotensin II (Ang II) receptor AT2R and novel signaling networks of the AT2R that can mitigate the progression of heart disease and vascular damage caused by diabetes and obesity.
Current projects include:

Heart failure with preserved ejection fraction (HFpEF) is a fatal syndrome with complex cardiopulmonary pathology and accounts for 40% of heart failure cases. There are no drugs that can treat HFpEF effectively. Obesity and diabetes and female sex increase the risk for HFpEF, however, what causes the sex differences in HFpEF is not understood. We have characterized heart disease progression in rat models for obesity and prediabetes and discovered new biomarkers for sex differences in heart disease progression. We have now developed a new female model for HFpEF and metabolic diseases. We are now testing the safety and efficacy of FDA-approved and experimental drugs in treating HFpEF using this model.
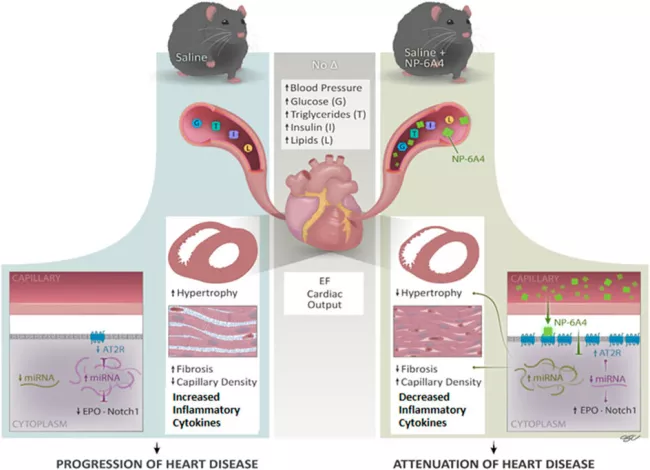
We are working to validate the cardiovascular and neuroprotective effects of NP-6A4, a new AT2R peptide agonist and a cardiomyopathy drug that has the FDA designation (from Novopyxis Inc). Pulakat lab showed for the first time that NP-6A4 is more effective than β- adrenergic receptor blockers, AT1R antagonist losartan, and AT2R agonist CGP42112A in protecting human cardiovascular cells from acute nutrient deficiency and NP-6A4 suppresses vascular stiffness. NIH-funded projects are directed to validate the efficacy and utility of NP-6A4 in effecting cardio-pulmonary protection via AT2R-specific novel immunomodulatory networks in conditions of severe acute or chronic inflammation.
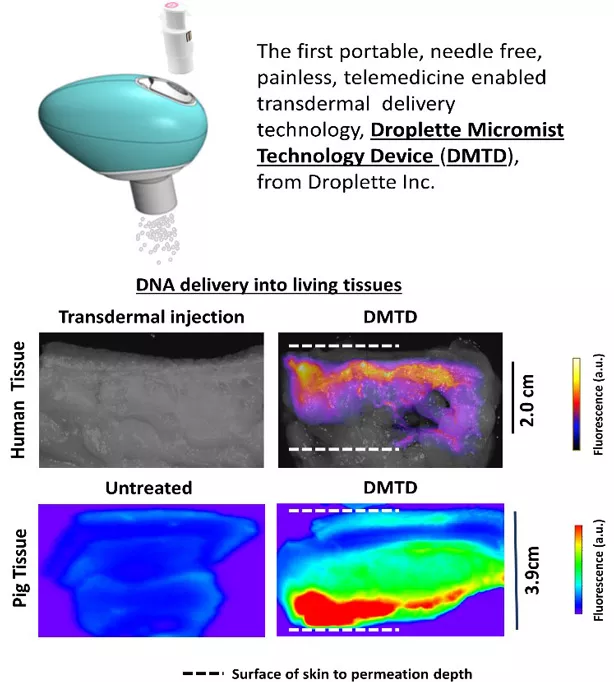
We are working to validate the use of a new transdermal delivery platform technology (from Droplette Inc.) to expedite the healing of chronic wounds (diabetic ulcers, pressure ulcers) via localized deep tissue delivery of growth factors and gene therapy. Our studies to evaluate the safety and efficacy of new gene and protein therapies to treat non-healing pressure ulcers that damage muscle and bone via localized delivery using custom-made Droplette Micromist Technology Devices (DMTDs) are supported by a DoD Technology and Therapeutic Development Award and Droplette Inc. Another collaborative project with Dr. Hongmin Sun at the University of Missouri and supported by a DoD grant is focused on evaluating the safety and efficacy of localized delivery of high molecular weight antibiotics by DMTD to treat deep skin tissue infection by multidrug-resistant bacteria.
Lab members
- Supriya Agrawal, Research Assistant
- Francisco Velasquez, Research Assistant