The Good Lab takes an interdisciplinary approach encompassing fundamental molecular and cellular biology, in vivo state-of-the-art imaging technologies, ex vivo vascular function techniques, and in vivo pathophysiological models to further our understanding of how blood flow regulation and inflammation contribute to the etiology of neurological diseases and to dissect the role of arterial and venous endothelial Panx1 in ischemic stroke and Alzheimer’s Disease.
Current projects include:
We have found a dual role for endothelial Pannexin1 (Panx1) in the cerebral vasculature regulating cerebral arterial myogenic tone and venous inflammation. Our previous work demonstrated that deletion of endothelial cell (EC) Pannexin1 (Panx1), the primary nucleotide release channel, significantly reduces post-ischemic stroke infarct volume. We found that EC Panx1 is required for cerebral artery myogenic reactivity, which contributes to post-stroke CBF recovery, and that deletion of EC Panx1 reduces the number of infiltrating leukocytes following an ischemic stroke. Alternatively, we have recently found that overexpression of endothelial Panx1 results in significantly increased infarct volume due to impaired recovery of cerebral blood flow (CBF) and increased infiltration of leukocytes specifically in female mice. Together these data suggest that endothelial Panx1 contributes to post-ischemic stroke outcome through regulation of CBF recovery and exacerbation of neuroinflammation. Our future studies are examining the molecular mechanisms by which Panx1 contributes to both arterial and venous vascular functions following an ischemic stroke and identifying any sex-specific effects of Panx1 in these pathways.
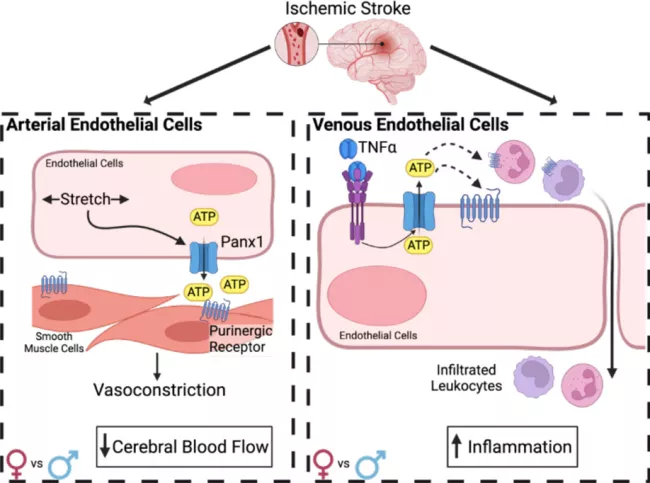
Published findings: Good et al, 2018 JCI Insight PMCID: PMC5926909
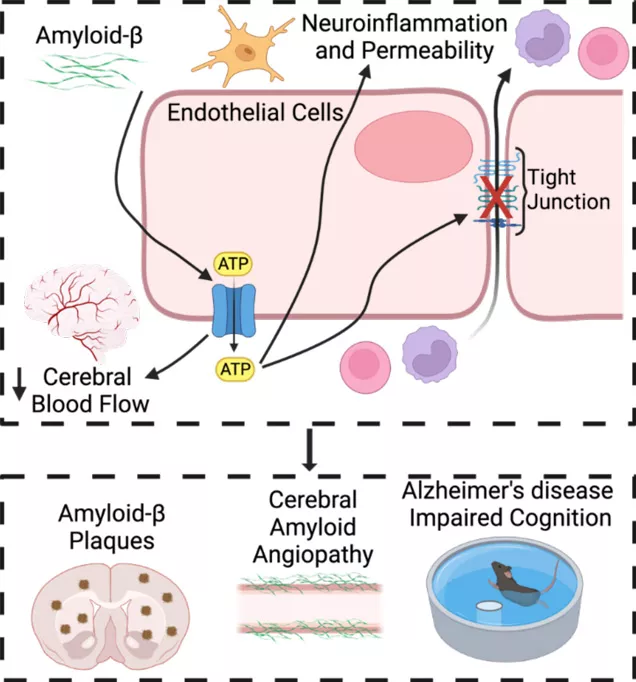
A new focus of the Good lab is understanding the contribution of vascular dysfunction to the development and progression of Alzheimer’s disease, which is currently funded by our recently awarded R21 grant from the NIA. Alzheimer’s disease (AD), which is a rising cause of mortality, is associated with vascular dysfunction. Progression into the symptomatic phase of AD is associated with impaired cerebral blood flow (CBF) as well as increased neuroinflammation and breakdown of the blood-brain barrier (BBB). Thus, identifying pathways that contribute to both CBF and neuroinflammation may lead to novel therapies that can intervene and prevent the progression of AD. We hypothesize that endothelial Panx1 promotes vascular dysfunction and cognitive decline in Alzheimer’s disease via increased vascular tone, neuroinflammation, and BBB breakdown.
Lab members
- Amanda Mauro, PhD, Postdoctoral research fellow
- Eric Dai, MS, Medical Student at Tufts University School of Medicine
- Mauricio Ruiz Soler, Rotating Tufts Master’s Student in Pharmacology and Drug Development
Previous members
- Colleen Duffy, MS, Research Assistant II 2020-2021 (currently Manager, Laboratory Equipment Procurement at Broad Institute of MIT and Harvard)
- Maria Tomàs Gracia, Research Assistant II 2020-2023 (currently a Medical Student at Yale University)
- Azita Shirinzadeh, Tufts University Undergraduate Researcher 2022