Current projects include:
We identified that the cGMP-dependent protein kinase 1 alpha (PKG1α) opposes pathologic cardiac remodeling through mechanisms that require the PKG1α leucine zipper protein interaction domain. Though PKG1-activating therapies remain under investigation for the treatment of heart failure, these agents have been limited by hypotension arising from PKG1-induced vasodilation. We have taken the strategy of screening for cardiac PKG1α leucine zipper binding substrates (Figure 1). We have used this strategy to delineate novel cardiac versus vascular-specific signaling mechanisms which might be selectively modulated in order to oppose LV remodeling but avoid excess hypotension. Our overarching hypothesis holds that myocardial PKG1α kinase substrates function as novel anti-remodeling molecules and therefore represent candidate therapeutic targets in cardiac remodeling and heart failure.
Current work in the lab focuses on understanding the role of newly discovered PKG1α - substrates molecules in opposing pathological cardiac remodeling. We carry out these studies in vitro, in cultured cardiac myocytes, and in vivo models to determine how these signaling molecules inhibit cardiac remodeling and to what degree these effects require PKGIα (Figure 1).
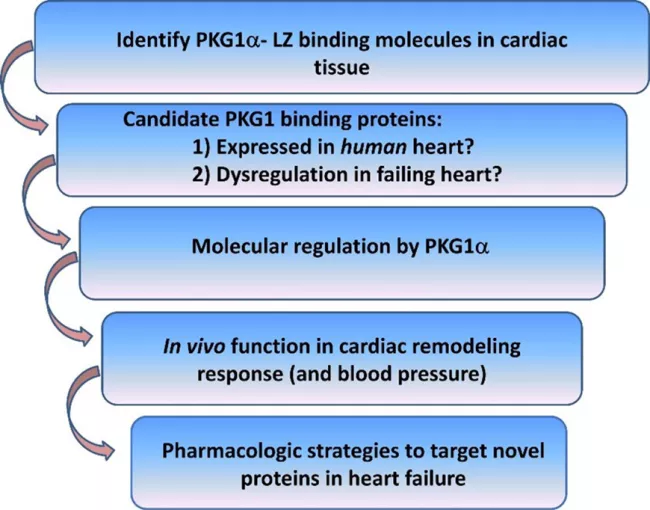
We also explore established and investigational PKG1-activating drugs in preclinical models of heart failure, to discover novel mechanisms of action, and to identify candidate therapies for heart failure.
Learn more about this work by reading: Thoonen et al, 2016; Richards et al, 2021
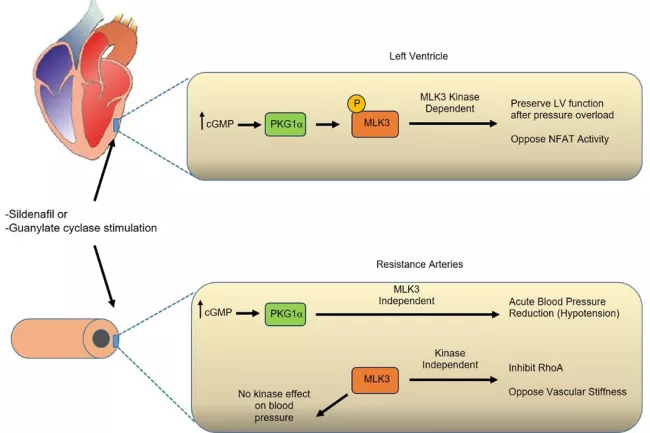
Our discovery strategy identified the protein Mixed Lineage Kinase 3 (MLK3) as a PKG1α substrate that opposes pressure overload-induced left ventricular dysfunction and also regulates blood pressure. Our working model holds that MLK3 modulates cardiac function through its kinase-dependent effects, whereas MLK3 controls blood pressure through non-kinase-mediated mechanisms. Current work in the lab is testing this model (Figure 2) using a variety of tissue-specific MLK3 deletion mutant mice and through genetic and pharmacological gain and loss of function studies.
Lab members
- Anna Burke, Masters’ Student
- Santo Kalathingal Anto, Postdoctoral Research Fellow
- Suchita Pande, Postdoctoral Research Fellow
- Hannah Elwell, MD, PhD Student